An effective treatment that’s made possible through research and dedication to continuous improvement
Innovation, integrity, leadership and a genuine care for our customers – these are the drivers behind what we do at 4CYTE™. We are a research and development company committed to pioneering joint health research and exploration in our field, leading us to create innovative, ground-breaking products that drive change within our industry.
Since 2008, 4CYTE™ has undergone rigorous assessments in both laboratory and clinical trials to test its safety and efficacy. Many of these studies have been peer reviewed and published in leading veterinary journals with Epiitalis® demonstrating not only symptom modifying capabilities, but also disease modifying effects. 4CYTE™ has also been scientifically proven to be as effective as a NSAID in treating osteoarthritis symptoms such as pain and inflammation.
Below, you’ll find a selection of our scientific highlights from a growing body of evidence that supports the safety and efficacy of 4CYTE™ and Epiitalis®. These excerpts have been highly summarised. If you are interested in learning, please visit our FAQ page or get in touch with our team.

Canine Science
A new leash on life: 4CYTE™ Epiitalis® Forte prevents and treats symptoms of osteoarthritis in dogs
The study
A pilot study of 4CYTE™ Epiitalis® Forte for Dogs, a novel nutraceutical, in the management of naturally occurring osteoarthritis in dogs
T Beths,* R Munn, SH Bauquier, P Mitchell and T Whittem
of dogs who used 4CYTE™ showed improvement in their quality of life*
94%of dogs who used 4CYTE™ showed an improvement in movement and also showed an improvement in quality of life.*
74%of dogs who used 4CYTE™ showed improvement in movement*
71%of dogs using prescription OA medications showed further improvement when 4CYTE™ was added to their treatment regime*
In a peer reviewed and published clinical trial conducted at Melbourne University in 2020, Epiitalis® Forte Gel for dogs was assessed in a one-month pilot study involving 46 dogs with naturally occurring osteoarthritis.
Two data collection tools were used. Gait analysis using the GaitRite walkway system collected objective data by measuring paw strike as the dogs walked over a 5m mat with sensors. The sorest limb, or paw exerting least pressure was identified as the study limb. Dogs completed 3 “passes” across the mat. The Helsinki Chronic Pain Index (HCPI) which asked a series of 11 quality of life-based questions, collected subjective data.
Study dogs were assessed on Day 0 and then sent home with Epiitalis® Forte Gel. Dogs returned 28 days later to complete their 2nd assessment.
At day 28:
- 74% of dogs improved the pressure placed on the sore limb, suggesting that there had been a reduction in pain.
- 93.5% of dogs improved their “Quality of life” score from the HCPI assessment, indicating reduction in pain and improvement in demeanour.
- 71% of dogs that were on concurrent osteoarthritis medication throughout the study, improved further.
This trial suggests that 4CYTE™ Epiitalis® Forte for Dogs successfully reduced symptoms of osteoarthritis in dogs.
4CYTE™ Canine Granules is as efficacious as NSAIDS
The study
A randomised controlled masked clinical trial of two treatments for osteoarthritis in dogs.
T Whittem,* L Richards, J Alexander, C Beck, C Knight, M Milne, M Rockman, R Saunders and D Tyrr

66 dogs participated in this study conducted at Melbourne University. Dogs were randomly allocated to receive either 4CYTE™ Canine Granules or reference product Carprofen as a positive control.
Dogs were assessed using 3 assessment tools, by veterinarians and owners on day 0, day 14 and day 28.
4CYTE™ Canine Granules was found to be statistically not inferior to Carprofen at Day 14 using the Owner Lameness score, and not inferior by day 28 using all 3 assessment scores. The results strongly support the conclusion that 4CYTE™ Canine Granules is not inferior to Carprofen, and that 4CYTE™ Canine Granules can be declared as bioequivalent to Carprofen by end-point efficacy.
In a peer reviewed and published study, 4CYTE™ Canine Granules demonstrated bioequivalence to Carprofen in canine osteoarthritis symptom reduction.
Equine Science
Epiitalis® demonstrates disease modifying effects in equine osteoarthritis study – a world first, scientific breakthrough
The study
Examining the effects of an extract of Epiitalis® in the osteochondral fragment-exercise model of osteoarthritis.
Kathryn A. Seabaugh, Myra F. Barrett, C. Wayne McIlwraith, David D. Frisbie.
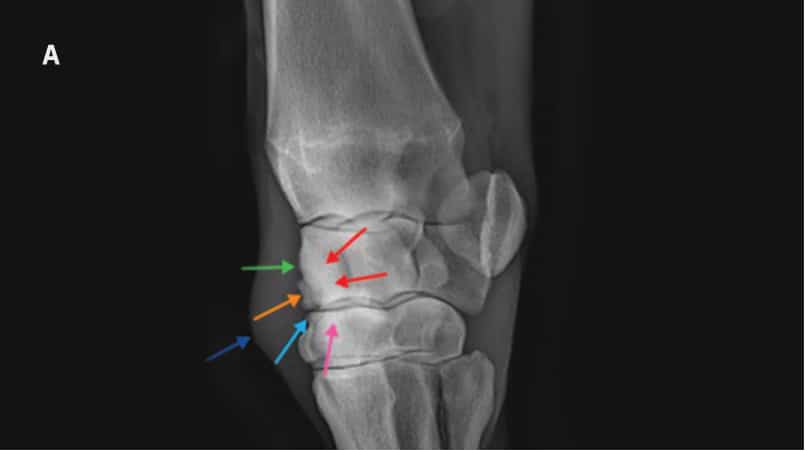
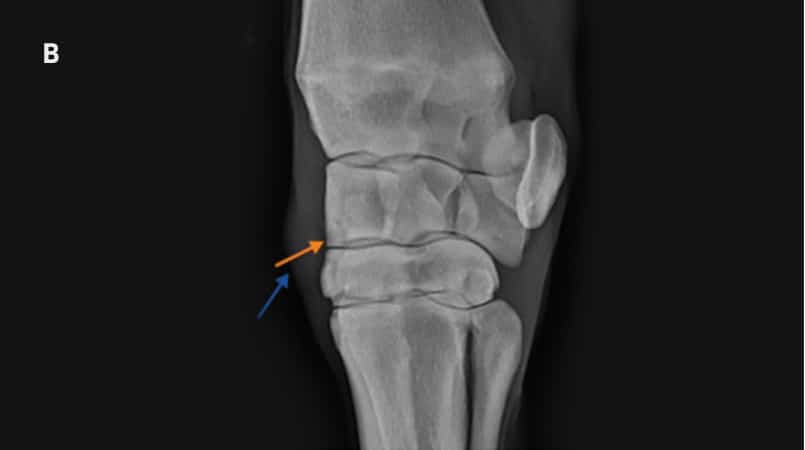
Dorsolatero-palmaromedial oblique radiographs of a placebotreated horse (A) and Epiitalis® treated horse (B) on Day 70.
Osteochondral fragment visible in both horses. Sclerosis of third carpal bone and radiocarpal bone are visible in placebo-treated treated horse as well as enthesopathy of the joint capsule, and osteophytosis. Soft tissue swelling representing middle carpal joint effusion is the only common abnormality between the two horses.
An assessment of the efficacy of Epiitalis® in a surgically induced equine model of OA demonstrated significant symptom-modifying and disease-modifying effects following oral treatment with Epiitalis®. Not only was potent management of local inflammation observed, but also obvious amelioration of X-ray measured OA-changes with a significant decrease in radiographic evidence of osteoarthritis.
Significant symptom-modifying and disease-modifying effects were seen following oral treatment with Epiitalis® in this equine in vivo model of osteoarthritis. The most significant results were related to the decreased concentration of PGE2 in synovial fluid and amelioration of radiographic changes in horses treated with 4CYTE™ Epiitalis® Forte for Horses.
Preventing inflammation with 4CYTE™ Equine Granules
The study
Evaluation of inflammatory responses induced via intra-articular injection of interleukin-1 in horses receiving a dietary nutraceutical and assessment of the clinical effects of long-term nutraceutical administration.
Wendy Pearson, PhD; Michael W. Orth, PhD; Michael I. Lindinger, PhD
By day 1.3, 4CYTE™ Equine fed horses’ synovial fluid PGE2 concentration was 45.4% lower than control
Part 1: Horses were fed 4CYTE™ Equine Granules at dosages of 0, 15g, 45g or 75g for 84 days to establish safety.
Part 2: One knee joint in each horse was injected with Il-1 on day 0 of the study. Synovial fluid Prostaglandin E2 (PGE2), Glycosaminoglycans (GAGs) and Nitric Oxide (NO) were analysed before, and at intervals following administration of the IL-1 injection.
In a clinical trial conducted at Guelph University, after administration of IL-1 to the knee joint, horses receiving 4CYTE™ Equine Granules had significantly lower PGE2, 32 hours after administration than horses not receiving 4CYTE™.
Joint circumference measures in the 4CYTE™ group were significantly improved compared to the control group.
This trial suggests that administration of 4CYTE™ may be beneficial in preventing inflammation associated with naturally occurring osteoarthritis and degenerative joint disease.
Improvement in just 1 week with 4CYTE™ Epiitalis® Forte for Horses
The study
To establish the clinical efficacy of dose rates in Epiitalis® in a gel oral application and its ability to reduce arthritis symptoms | Dr Frederick W. Benker et al, Equine Medical Centre of Ocala
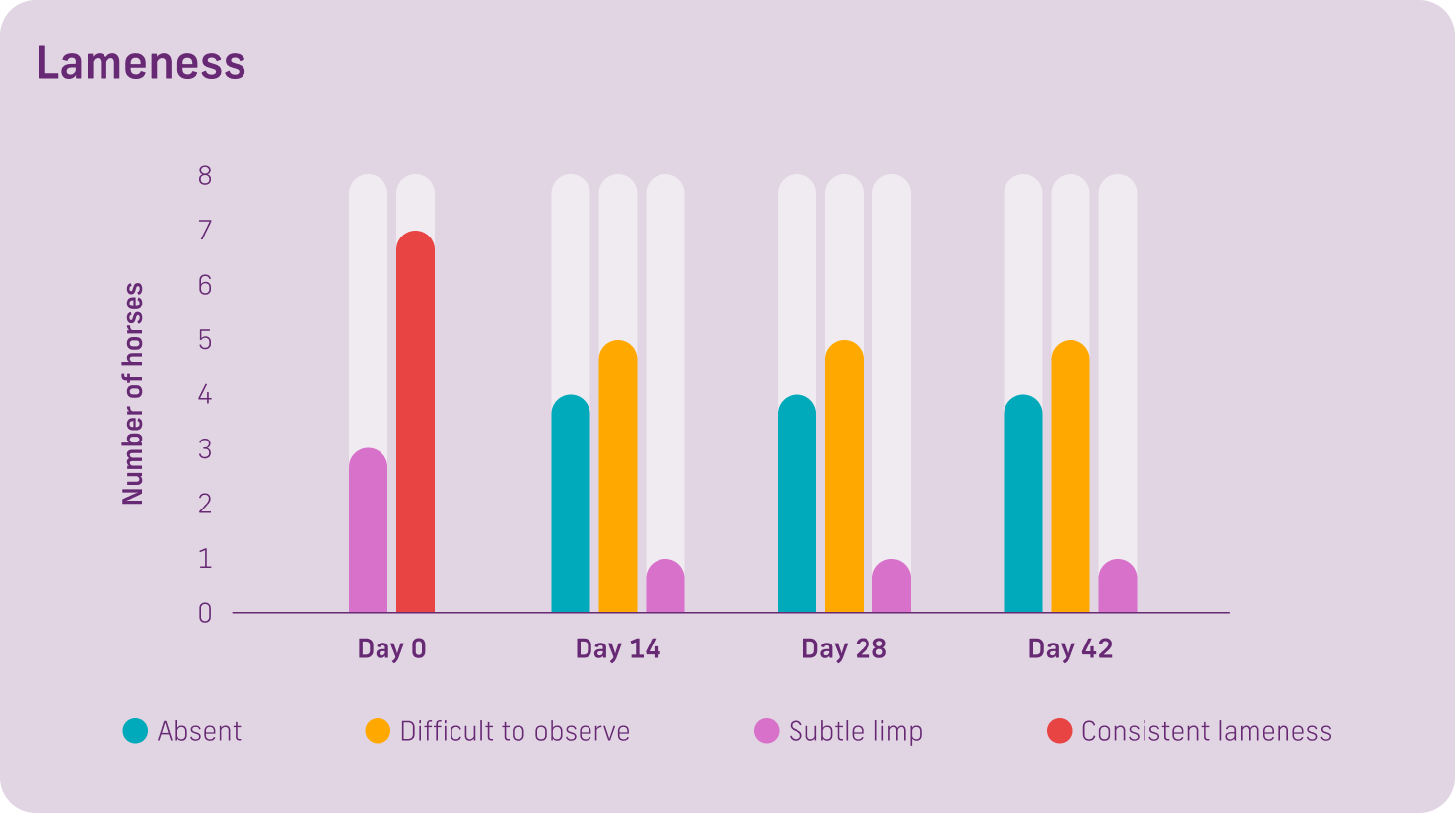
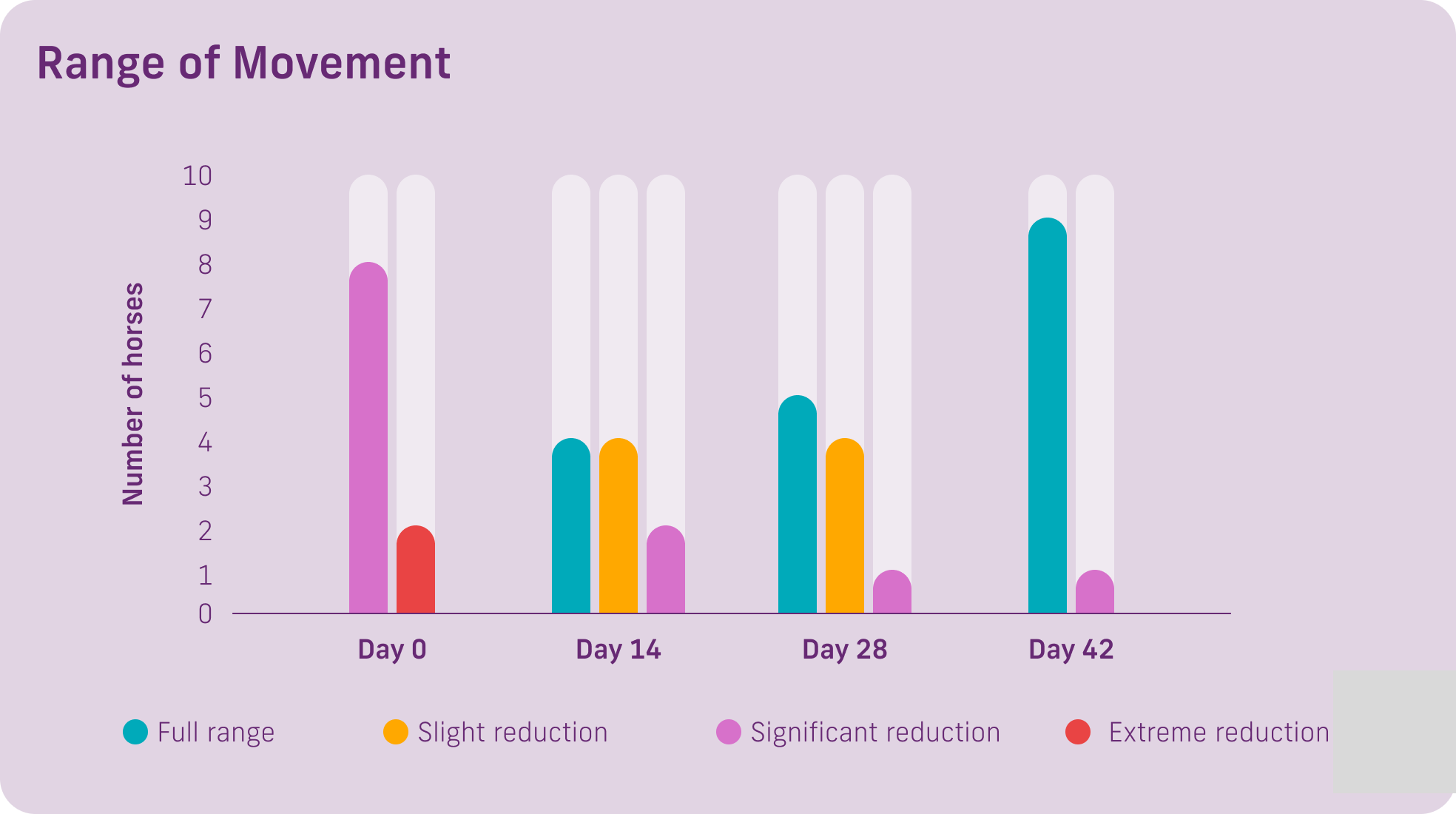
Ten horses were selected showing signs of arthritis or joint effusion. They were randomly allocated to two treatment groups for 42 days. One group received 8g from day 1-14, then 4g from day 15-42. The second group received 8g from day 1-28 then 4g from day 29-42.
Lameness: 7 of the 10 horses had a consistent lameness at day 0. 8 of the 10 horses at day 14 had an absent or difficult to observe lameness. Only 1 horse had a subtle limp after 42 days.
Heat and Swelling: 6 of the 10 horses had significant heat and swelling at Day 0. 8 of the 10 horses were only showing nominal to absent levels after 14 days.
Range of Movement: 8 of 10 horses had a significant reduction in range of movement/mobility at Day 0. At day 14, only 8 horses were showing full range or a slight reduction in range of movement/mobility. At Day 42, 9 of the 10 horses had full range of movement mobility.
Within one week of treatment the levels of improvement were hugely significant in all 9 of the horses.
